Interviewed and edited by Sarah Anderson, PhD
When COVID-19 reached Canada in 2020, the Jean lab was ready. MBIM professor and virologist Dr. François Jean, founder and former scientific director of UBC’s Facility for Infectious Disease and Epidemic Research (FINDER), focuses on the discovery and preclinical investigation of antivirals against human pathogenic “enveloped” viruses, which are packaged within a lipid membrane. When the SARS-CoV-1 outbreak hit in 2002, Dr. Jean’s team developed screening platforms to identify anti-SARS-CoV lead compounds. “When SARS-CoV-2 struck, we revisited a lot of the virological and molecular tools that we had developed back then,” Dr. Jean said. “We were prepared for this because we had invested time and effort 15 years ago in coronavirus research and the establishment of a state-of-the art containment level-3 facility at UBC to respond to emerging and re-emerging infectious disease threats.”
In redirecting his knowledge and experience toward SARS-CoV-2, Dr. Jean became the lead of Pillar 10, the antiviral strategies and therapeutics pillar of the Coronavirus Variants Rapid Response Network. Within that network, his team worked in collaboration with UBC chemistry professor Dr. Raymond Andersen to discover antiviral molecules from natural sources such as a British Columbia sea sponge, co-developed a potent antiviral compound formulated as a nasal spray, and explored resistance mechanisms associated with currently approved SARS-CoV-2 antiviral drugs. Now, five years after the COVID-19 pandemic was declared, Dr. Jean reflects on his team’s progress, the state of COVID-19 research, and lessons learned that can improve pandemic preparedness and response.

The public health response to COVID-19 largely focused on vaccines. What is the importance of antivirals as a complementary approach?
Antiviral drugs are extremely important for treating infection regardless of a person’s vaccination status. A 2024 retrospective study of hospitalized patients with COVID-19 found that the combined use of vaccine and oral antiviral drugs was associated with substantially reduced mortality and disease progression. However, the recent SARS-CoV-2 Omicron variants display mutations that increase their ability to evade immunity from neutralizing antibodies, whether produced by natural infection or vaccination.
To overcome this limitation, my team has spearheaded the development of host-directed antivirals (HDAs) for SARS-CoV-2. HDAs target key cellular proteins and functions in the host that are commonly hijacked by human enveloped viruses to support their lifecycle; these are not the viral proteins that are subject to high mutation rates. By acting on host factors that are exploited by a wide range of pathogenic viruses, HDAs represent a very promising class of broad-spectrum therapeutics that are effective regardless of the emerging or re-emerging virus. We are currently leading preclinical studies on three novel classes of broad-spectrum HDAs that act on three different human host factors: transmembrane protease, serine 2 (TMPRSS2), vacuolar-type ATPase (V-ATPase), and protein kinase C (PKC). These lead antivirals should still robustly inhibit viral infection in human cells even as the virus continues to mutate in humans.
You recently published a study on N-0920, a new host-directed antiviral compound that treats COVID-19. Could you discuss that work?
One attractive target for our HDA strategy is human TMPRSS2, an enzyme that cleaves viral surface proteins such as the SARS-CoV-2 spike protein, thus allowing the virus to enter the cell and release its genetic material. In our 2022 study published in Nature, we identified a TMPRSS2 inhibitor, N-0385, and demonstrated that it successfully blocked cellular entry of Alpha, Beta, Gamma, and Delta SARS-CoV-2 variants of concern in human lung cells and reduced the severity of SARS-CoV-2 infections (both the original strain and Delta variant of concern) when administered intranasally to mice. With activity at nanomolar-range concentrations, N-0385 was at the time the most potent HDA discovered to date, and I didn’t think we could make that drug better. However, we collaborated with the medicinal chemistry team at the University of Sherbrooke led by Professor Pierre-Luc Boudreault, who synthesized a library of 135 N-0385 analogs. In our new study published in the Journal of Medicinal Chemistry, we reported the discovery of N-0920, an N-0385 analog with exceptional potency in reducing SARS-CoV-2 omicron variants’ entry in human lung cells. N-0920 represents the first picomolar antiviral lead drug for circulating SARS-CoV-2 omicron variants.
Importantly, these TMPRSS2 inhibitors have shown no signs of toxicity in human lung cells. The concern with HDAs is that disrupting the activity of a cellular protein could impair the functionality of the cell. However, researchers have demonstrated that knocking out the TMPRSS2 gene in a mouse model has no side effects, suggesting that the enzyme is not critical for maintaining a healthy cell. In fact, when we tested the compounds in human lung cells, we observed no cytotoxicity even when using the drug at 1 million times the concentration needed to block the infection. Our findings provide early evidence that it is feasible to target cellular factors without compromising the host, opening up the door for other host-based therapeutic interventions.
Why is it important that the scientific community remains invested in COVID-19 research in 2025?
Despite the public narrative that COVID-19 is no longer a concern, the SARS-CoV-2 virus is not gone; in fact, highly immune-evasive omicron variants continue to circulate around the world and cause illness and death. Right now, there is a complex dynamic emerging related to the co-circulation of SARS-CoV-2 and other respiratory viruses such as influenza A and respiratory syncytial virus (RSV). The airway epithelial cells in the human upper respiratory tract are susceptible to all three of these viruses, and cross-talk between them has opened up both an ongoing health threat and an interesting area of study into co-infection and tripledemics.
Furthermore, millions of people are still grappling with long-term effects of acute SARS-CoV-2 infection, and they depend on continued research into the mechanisms, epidemiology, prevention, and treatment of long COVID. Overall, there is growing evidence that antivirals for SARS-CoV-2 play a role in preventing long COVID, and the hypothesis that patients with established long COVID could benefit from antiviral therapy is both plausible and worthy of investigation. Our lab is currently exploring the neuroinvasive potential of SARS-CoV-2 to better understand “brain fog” — a debilitating symptom of long COVID in which patients struggle with cognition, memory, and focus. Using astrocytes derived from a patient’s central nervous system and mini brain organoids, we are examining how the virus hijacks astrocytes and how that impacts neurological function.
Your team contributed a chapter to a new pandemic guidebook. Reflecting on the past five years, what lessons learned can improve pandemic preparedness and response?
To better prepare for future pandemics, it is critical that we invest in developing broad-spectrum antivirals so that regardless of which viruses arise or begin to propagate, we have access to life-saving drugs. As a global health priority, we need to embrace host-directed antiviral strategies that will provide desperately needed broad-spectrum therapeutics against current and emerging viral pathogens.
We have learned that direct-acting antivirals (DAAs) that combat specific viruses carry the risk of increasing antiviral resistance, particularly when they are administered alone as a monotherapy. In a recent study published in The Journal of Infectious Diseases in collaboration with Dr. Robert Kozak at the Sunnybrook Health Science Centre in Toronto, we analyzed samples from patients treated with the DAA nirmatrelvir/ritonavir (paxlovid) and identified antiviral resistance mutations in the drug target: 3C-like protease (3CLpro), a SARS-CoV-2 enzyme involved in virus replication. HDAs are less susceptible to drug resistance compared to DAAs because, unlike viral genes targeted by DAAs, host genes targeted by HDAs have a low propensity to mutate. Therefore, HDAs and DAAs could be administered in combination in an effort to overcome monotherapy resistance to DAAs and provide a more effective treatment through potential synergistic activity of the drugs.
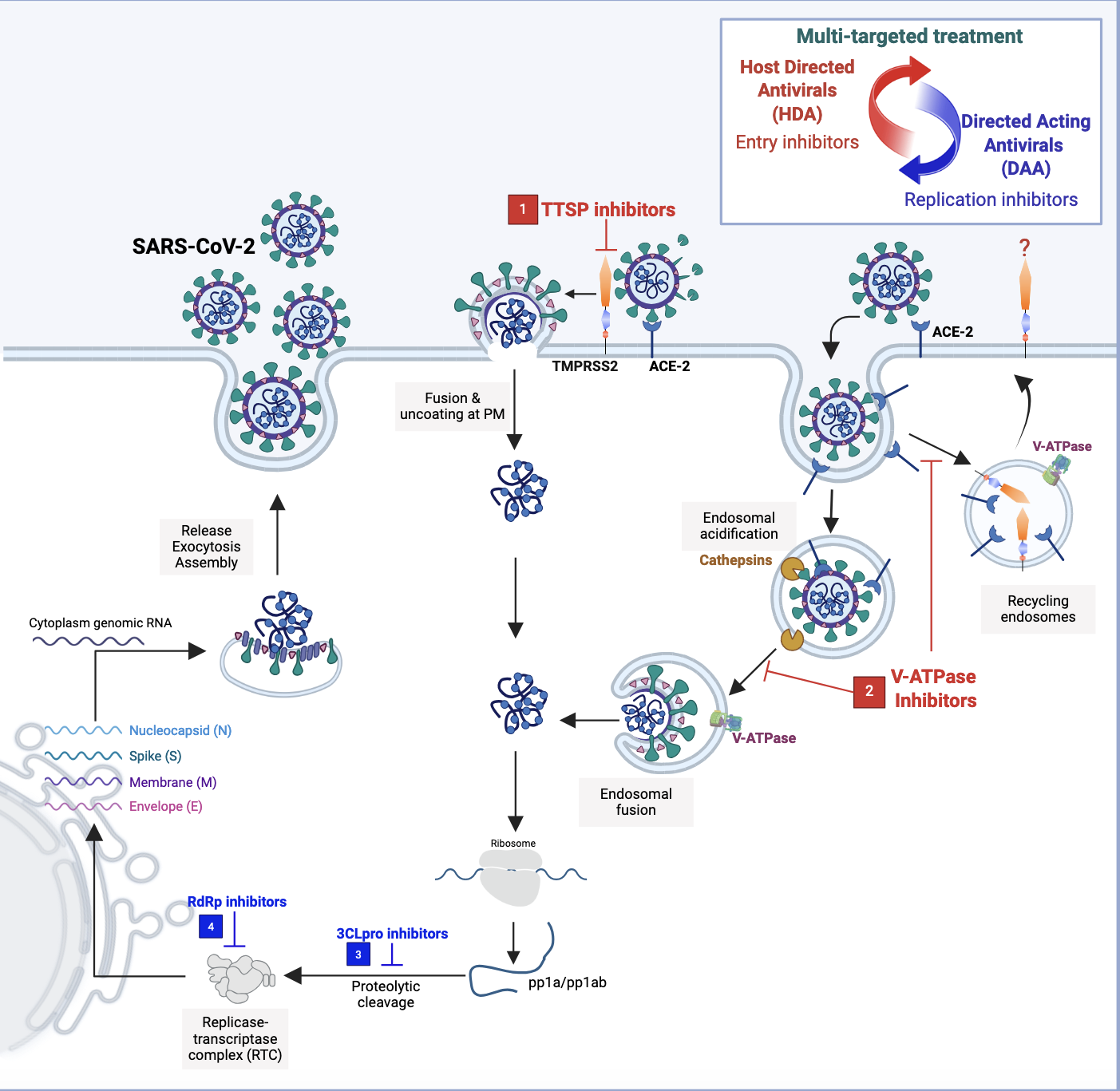
This collaborative study also highlights the importance of integrating clinical data into investigations of antiviral resistance. When we culture human cell lines infected with the SARS-CoV-2 virus in the presence of paxlovid repeatedly over time in a petri dish, we see that mutations appear in the 3CLpro enzyme’s catalytic site where the drug binds. But in the patient population, the mutations occur far from the catalytic site, leaving the protease activity intact while still conferring resistance to the antiviral compound. This finding suggests that the viral evolutionary path in human patients is distinct from the one virologists observe in the lab. Therefore, we must continue to monitor paxlovid-treated patients to discover novel resistance mutations that will inform the development of new therapeutics, treatment protocols, and strategies to mitigate viral resistance.
More broadly, we have learned that pandemic preparedness programs need continuous financial support from the Canadian government. Funding for the Coronavirus Variants Rapid Response Network was terminated on March 31, 2025, and it is disappointing to see this program shut down when we are on the cusp of another pandemic: avian influenza. I remember when SARS-CoV-1 struck, people said, “This is one of a kind; it will never happen again.” But 15 years later, we got hit by COVID-19, which was even worse. So rather than operating in emergency response mode each time a new viral threat emerges, we need to maintain the momentum and capitalize on the progress from the last pandemic by keeping national and international research networks and resources open and active. This will support the discovery of novel broad-spectrum lead antivirals, the preclinical development of antiviral drugs, and the training and mentorship of desperately needed Canadian scientists to combat global threats from current, new, and re-emerging infectious diseases.